
If an atom has more electrons than protons, then it has an overall negative charge, and is called a negative ion (or anion). If the numbers of protons and electrons are equal, as they normally are, then the atom is electrically neutral. Each proton has a positive electric charge, while each electron has a negative charge, and the neutrons, if any are present, have no electric charge. More than 99.94% of an atom's mass is in the nucleus. Atoms are so small that accurately predicting their behavior using classical physics is not possible due to quantum effects. This is smaller than the shortest wavelength of visible light, which means humans cannot see atoms with conventional microscopes. A human hair is about a million carbon atoms wide. The number of neutrons defines the isotope of the element.Ītoms are extremely small, typically around 100 picometers across. For example, any atom that contains 11 protons is sodium, and any atom that contains 29 protons is copper. The atom is the basic particle of the chemical elements, and the chemical elements are distinguished from each other by the number of protons that are in their atoms.
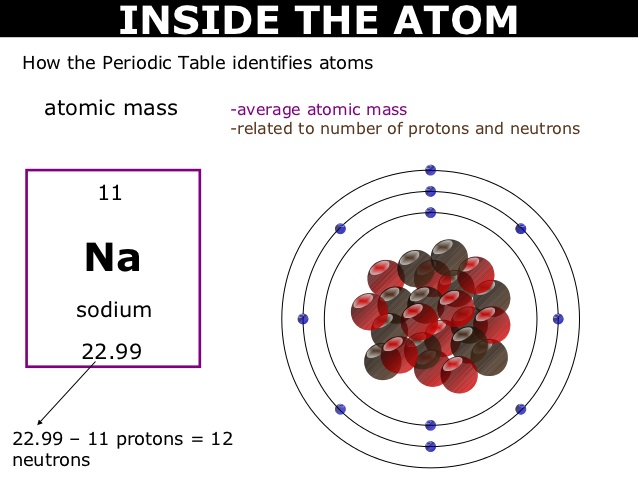
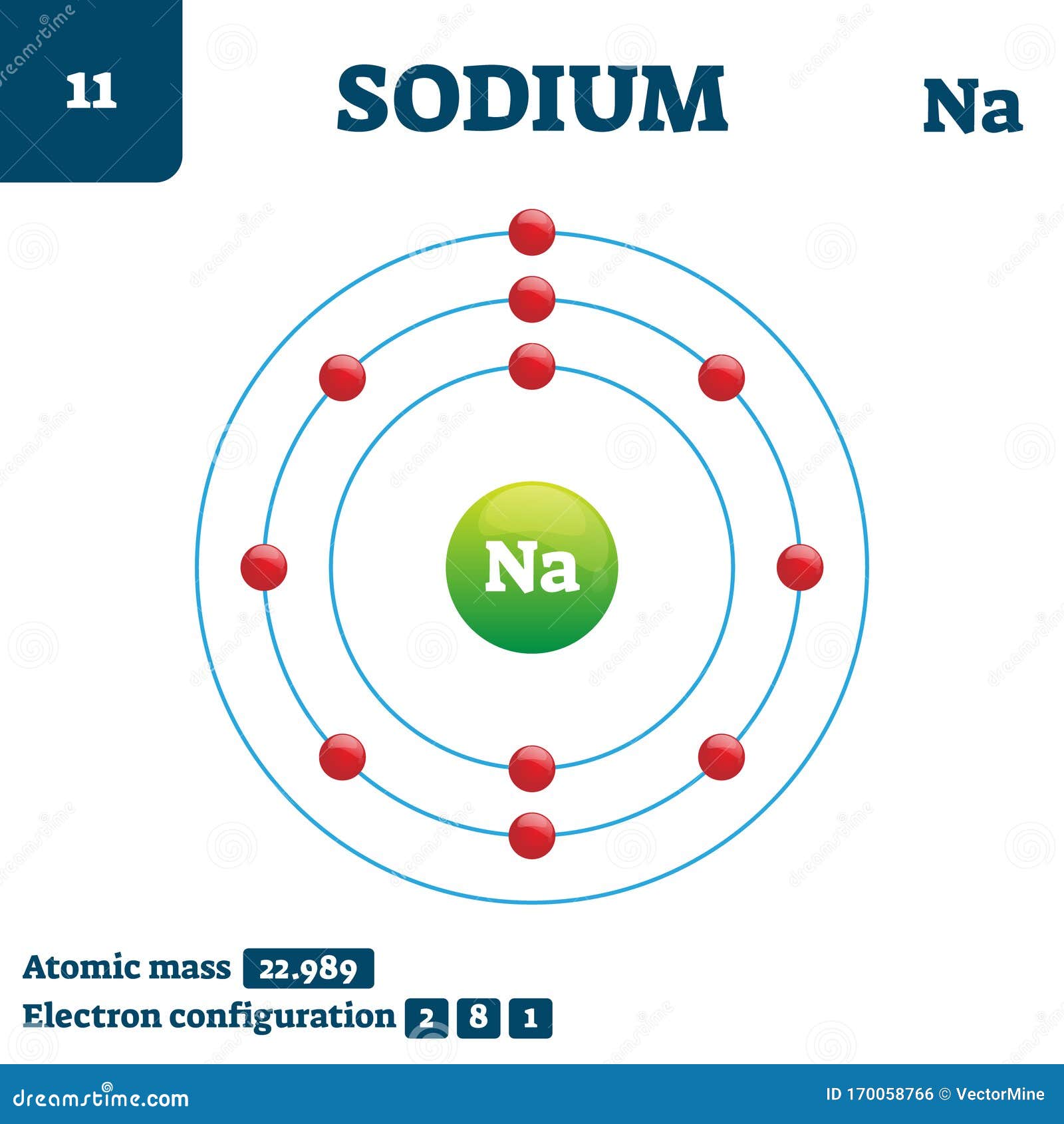
Smallest recognized division of a chemical elementĮlectrons and a compact nucleus of protons and neutronsĪn atom is a particle that consists of a nucleus of protons and neutrons surrounded by an electromagnetically-bound cloud of electrons. The black bar is one angstrom ( 10 −10 m or 100 pm). The nucleus (upper right) in helium-4 is in reality spherically symmetric and closely resembles the electron cloud, although for more complicated nuclei this is not always the case. An illustration of the helium atom, depicting the nucleus (pink) and the electron cloud distribution (black).


 0 kommentar(er)
0 kommentar(er)
